Roberta Tittarelli,1 Giulio Mannocchi,1 Flaminia Pantano,1 and Francesco Saverio Romolo1,2,*
Go to:
Abstract
The definition New psychoactive substances (NPS) refers to emerging drugs whose chemical structures are similar to other psychoactive compounds but not identical, representing a “legal” alternative to internationally controlled drugs. There are many categories of NPS, such as synthetic cannabinoids, synthetic cathinones, phenylethylamines, piperazines, ketamine derivatives and tryptamines. Tryptamines are naturally occurring compounds, which can derive from the amino acid tryptophan by several biosynthetic pathways: their structure is a combination of a benzene ring and a pyrrole ring, with the addition of a 2-carbon side chain. Tryptamines include serotonin and melatonin as well as other compounds known for their hallucinogenic properties, such as psilocybin in ‘Magic mushrooms’ and dimethyltryptamine (DMT) in Ayahuasca brews.
Aim:
To review the scientific literature regarding tryptamines and their derivatives, providing a summary of all the available information about the structure of these compounds, their effects in relationship with the routes of administration, their pharmacology and toxicity, including articles reporting cases of death related to intake of these substances.
Methods:
A comprehensive review of the published scientific literature was performed, using also non peer-reviewed information sources, such as books, government publications and drug user web fora.
Conclusions:
Information from Internet and from published scientific literature, organized in the way we proposed in this review, provides an effective tool for specialists facing the emerging NPS threat to public health and public security, including the personnel working in Emergency Department.
Keywords: Clinical effects, Emergency Departments, Fatalities, Forensic Toxicology, Intoxication, New Psychoactive Substances (NPS), Tryptamines.
Go to:
INTRODUCTION
The New psychoactive substances (NPS) are defined by European Community (2005) as “substances of abuse either in a pure form or preparation that are not controlled by the 1961 Single Convention on Narcotics Drugs or the 1971 Convention on Psychotropic Substances, but which may pose a public health threat comparable to scheduled substances” [1]. According to Zuba, “they are created usually by manipulating already existing psychoactive substances or, less, by finding drugs with a new chemical structure that may produce similar effects to those of the illegal drugs of abuse” [2]. They may be formulated as “bulk powders, liquids, tablets, capsules, blotters,” or herbal preparations, indeed on the web sites these drugs are sold as “research chemicals,” “incense,” “bath salts,” or “plant-growth fertilizers,” equipped with labels specifying that the products are “not for human consumption” or “not for sale to minors” [3]. The International Narcotics Control Boardregards the Internet “as a growing source of on-line drug trafficking”: products purchased on Internet may vary over time proposing chemicals always different, not listed in literature, meaning that users are often unaware of what or how much they are taking [4], causing increasing risks to public health. The phenomenon of marketing illicit drug ‘‘derivatives’’ grew mainly because of their easy availability on the Internet or in “head shops”. The use of NPS has increased dramatically in recent years: only in the European Union, the number of NPS identified has risen “from 14 at the end of 2005 to 236 by the end of 2012” [5]. The majority of new recreational synthetic drugs fall into one of the following psychoactive drug categories: synthetic cannabinoids, synthetic cathinones, phenylethylamines, piperazines, ketamine derivatives and tryptamines.
Tryptamines are a group of monoamine alkaloids (Fig.
1
1), which can be synthesized by decarboxylation of the aminoacid tryptophan: they can be found in plants, fungi, animals, microbes and amphibia [6].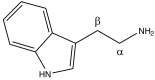
| Fig. (1) |
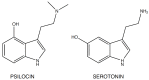
The tryptamines play a fundamental role in human life: serotonin (5-hydroxytryptamine, 5-HT), one of the most important signaling hormones [7] in the body, is a tryptamine natural derivative involved in regulation and modulation of multiple processes within the central nervous system, such as sleep, cognition, memory, temperature regulation and behavior [8]. Moreover, mammalian brain contains very low concentrations (in the low ng/g range) of tryptamine, which may act as a neurotransmitter or modulator [9]: it acts as a serotonin releasing agent and it is an enhancer of serotonergic activity [10]. Gibbons in his publication, defines the nature “an astounding chemist (..) able to produce compounds that have profound effects on the central nervous system (CNS)”[11]. Naturally occurring derivatives of the tryptamines are present in the ‘Magic mushrooms’ belonging to Psilocybe cubensis species, which contain psilocybin (4-phosphoryloxy-N,N-dimethyltryptamine) and psilocin (4-hydroxy-N,N-dimethyltryptamine), the latter showing marked similarities with serotonin (Fig.
2
2).
| Fig. (2) |
3
3).
| Fig. (3) |
Go to:
CLASSIFICATION OF TRYPTAMINES BASED ON THEIR CHEMICAL STRUCTURES
Several classifications have been suggested for these substances. Nichols classified tryptamines in two main groups: -the simple tryptamines, including dimethyltryptamine (DMT), and the ergolines (a group of chemical compounds that were originally synthesized from a fungus ergot, among which is the lysergic acid diethylamide (LSD). Fantegrossi divided tryptamines in three subgroups: (1) simple tryptamines, without modification of the indole ring; (2) tryptamines having a modification on the 4-position on the indole ring; (3) tryptamines having a modification on the 5-position. Only substitutions on the 4-5 positions were considered because changes in position 6 or 7 result in reduced hallucinogenic activity [14] (Fig.
4
4).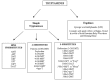
| Fig. (4) |
Classification of tryptaminesGo to:
STRUCTURE-ACTIVITY RELATIONSHIPS IN TRYPTAMINES AND THEIR METABOLISM
The effects caused by the administration of tryptamines are closely related to their structures, as each of these compounds has a different receptor affinity to which are related psychoactive phenomena.
Go to:
RING UNSUBSTITUTED COMPOUNDS
Several compounds belong to this class, including α-methyltryptamine (AMT, street name Spirals) and α-ethyltryptamine (AET) both showing stimulant activities [18].
Alpha-methyltryptamine (AMT) was firstly developed as an antidepressant agent called as INDOPAN, in 1960’s by the Upjohn Company and used for a short period of time in the former Soviet Union [20], but at last it was recognized as a toxic substance able to produce psychosis [21].
AMT activity is linked with the release of dopamine and its re-uptake inhibition. AMT also acts on serotonin and noradrenaline receptors and inhibits MAO activity in vitro and in vivo, therefore it is active after oral administration. Nagai [16] in his study, developed a method to determine the reuptake and release of monoamines (dopamine, serotonin and norepinephrine), and “analyzed the effects of various designer drugs on monoamine neurotransmission”, using rat brain synaptosomes. He disclosed that AMT presented the “strongest monoamine re-uptake inhibitory activity and release stimulating activity, although monoamine release was more dominant than re-uptake inhibition” [16]. Furthermore, he discovered that 5-MeO-AMT, “inhibited monoamine (dopamine, 5-HT and norepinephrine) re-uptake and stimulated monoamine release; its potency was second to that of AMT. AMT and 5-MeO-AMT both have a primary amine group, which in tryptamine might be indispensable for the stimulation of monoamine release” [16]. Illegal use of α-methyl-tryptamine is based on its hallucinogenic and psychedelic visual effects, with doses ranging from 15 to 30 mg if administered orally. It can be smoked (4-20 mg) as freebase, and snorted as well. The onset of action, if orally administered, is between 3 and 4 hours, generally lasting up to 12-24 hours, but in some subjects the action can last until 2 days. The onset of action if smoked is in the range of seconds or minutes. Uncomfortable feelings are reported, such as restlessness and irritability, hyperthermia, anorexia, vomiting, inability to sleep, tachycardia, increase in blood pressure pupils dilatation, deep tendon reflexes and coordination impairment [22]. Wilcox [23] interviewed 15 individuals who had used α-methyltryptamine. Most common side effects reported were anxiety, nausea and moderately severe dysphoria as well as depression (15% severely depressed mood on the day after). The 90% of patients reported visual hallucinations and 87 % euphoria. Anxiety was lamented in 35% of cases, in a correlation with crowded environment. AMT, as every psychedelic agent can alter judgement and perception and therefore it can cause dangerous behaviours. In the US, since 2004, it’s placed as a schedule I substance, while in the UK it was completely legal until June 2014 [24]. According to the NPSAD (National Program on Substance Deaths Abuse) Annual Report, from January to December 2012 in the UK, α-methyltryptamine has showed the highest number of deaths (2 reported in 2011 and 4 reported in 2012) compared to tryptamine or 5-methoxy-diallyltryptamine (5-MeO-DALT) [25].
Also alpha-ethyltryptamine (AET) was firstly introduced as an antidepressant agent by Upjohn company in 1960s with the name MONASE, but it was withdrawn from the market because of an unacceptable incidence of idiosyncratic agranulocytosis. AET may induce serotonin neurotoxicity [26], similar to that of MDMA and para-chloroamphetamine (PCA), as reported in studies on rats. AET inhibits MAO activity [27] in vitro and in vivo, as AMT, and it is orally active. Lessins [18] studied the effects of these two alpha-tryptamine derivatives in mice, observing marked stimulation with increased locomotor activity, hyperthermia, midriasis and generalized body tremor: AET assumption is also correlated with psychedelic, stimulant, and entactogenic effects.
The dosage reported by Shulgin, for oral administration, is 100-150 mg. and it seems to be able to fight opiate addiction in anecdotal reports. “It appears to serve well, with short term dosage regimens, as an effective tool in kicking dependency on opiates” [19].
Dimethyl-tryptamine (DMT) was first synthesized in 1931 by Richard Helmuth Fredrick Manske, but it was discovered as a natural product only in 1946, when a brazilian chemist and microbiologist, Oswaldo Gonçalves de Lima, isolated it from the root bark of Mimosa tenuiflora. Since 1955 DMT has been found in at least fifty species of plants belonging to ten families [28], in four species of animals, and in three species of mammals. The biosynthesis of DMT begins with the decarboxylation of tryptophan that leads to the production of tryptamine. Further, tryptamine undergoes a methylation process, generating the intermediate product N-methyltryptamine (NMT). NMT is in turn transmethylated to form the final product N,N-dimethyl tryptamine. DMT binds several serotonin receptors, acting as a partial agonist in particular on the 5-HT2A and 5-HT2C receptors. It has also been shown to possess affinity for the α1 and α2-adrenergic receptors, dopamine D1, and sigma-1 (σ1). The psychedelic activity of DMT, similar to LSD and mescaline, can probably be attributed to the binding to 5-HT2A receptor, although the activation of other receptors, such as 5-HT2C and sigma-1, may play a very important role in the onset of these effects [29]. DMT can be taken orally, intravenously or by inhalation. When inhaled, a standard dose of DMT is 15–60 mg. The onset of the effects is very fast, less than 45 seconds, peak effects are reached within a minute and they last usually for about 15 minutes. The experiences after injecting a DMT are similar to inhalation in duration, intensity and characteristics. If orally administered DMT must be associated with a MAOI, as it is deaminated by monoamine oxidase and is quickly inactivated. The Ayahuasca brew, used in religious rituals of the native peoples of the Amazon, is obtained by boiling the Ayahuasca vine (Banisteriopsis caapi), that contains monoamine oxidase inhibitors (ß-carbolines harmaline, harmine, and 1,2,3,4 tetrahydroharmine) with the leaves of shrubs of the genus Psychotria or Diplopterys, that contains DMT. A review on the risk assessment of the use of Ayahuasca has been made by Gable, who reported that to achieve the lethal dose in humans, it would be necessary to ingest more than 20 times the ceremonial dose. The brew seems also to have minimal dependence potential [30].
Diethyl-tryptamine (DET) has not been found to occur naturally and, although presenting structural similarity to DMT, it is active when administered orally, due to the presence of two ethyl groups linked to its nitrogen atom, preventing its degradation by MAO. Its mechanism of action is not clear, but, like other psychedelic tryptamines, DET could present an agonist activity towards serotonin receptors [31, 32]. When orally administered the dosage is 50–100 mg and the effects are reported to last about 2–4 hours [33].
Dipropyl-tryptamine (DPT) was firstly synthesized in the 1950s [34], but it was firstly reported for use in the scientific literature only in 1973 [35], as an adjunct in psychotherapy of alcoholics. It is found either as its crystalline hydrochloride salt or as an oily or crystalline base and, as DET, it has not been found to occur naturally. There are few peer-reviewed experimental studies that try to explain the ways of interaction among DPT and serotonin receptors: Nagai revealed a strong inhibition of 5-HT reuptake in rat synaptosomes [16], and Thiagaraj also observed a moderate affinity partial agonism at the human 5-HT1A receptor [34]. Experiences related to DPT assumption are mostly psychedelic sensations, such as an increase of music and colors intensity, the vision of pleasant flashes of light and sparkles, a complete ego loss, and apparitions of faces. The dosage of DPT, for oral administration, is 100-250 mg and the duration of the psychoactive effects varies from 2 to 4 hours.
Diisopropyl-tryptamine (DiPT) is a synthetic hallucinogen, structurally related to dimethyl-tryptamine, but, unlike DMT, which produces short term visual hallucinations, DiPT causes auditory distortions [36]. DiPT presents a similar molecular mechanism of action as other hallucinogens. It is an agonist at 5HT2A receptors and a partial agonist at 5HT1A receptors [37], but 5-HT1A activity “is not thought to be necessary for hallucinogenic effects” [38]. Furthermore, DiPT blocks the serotonin uptake and it has little interaction with dopamine or norepinephrine transporters [16].
Effective dosage, orally administered, ranges from 50 to 100 mg [39] and the hallucinogenic effects continue until 6-8 hours.
Go to:
4-POSITION MODIFIED TRYPTAMINES
Psilocin (4-OH-DMT) can be assumed “as the prototypical agent of the 4-position modified tryptamines” [15]. It is obtained by dephosphorylation in the body of psilocybin found in hallucinogenic mushrooms of the species Psilocybe and Stropharia. In P. cubensis, psilocybin percentage varies from 0.2% to 0.4% of the dry weight [40], while psilocin is only present in trace amounts. After ingestion, psilocybin undergoes rapid hydrolysis of the phosphate groups by the alkaline phosphatase and then turns into its active metabolite psilocin, representing the active drug of abuse [41]. After the ingestion of Psylocibe ‘Magic mushrooms’, the hallucinogenic effects arise within the first 2 hours and then decrease in the subsequent 3-4 hours to disappear within 4-8 hours. The threshold of intoxication is reached after the intake of 40 µg psilocybin/kg body weight; “ typically 1–2 g of dried mushroom is ingested”, which yields from 2 to 8 mg of psilocybin [42]. “Pharmacokinetic studies of psilocybin in humans have shown” that, after “the rapid dephosphorylation of psilocybin to psilocin”, there is a conversion in plasma of “the psilocin into 4-hydroxy-tryptophole (4HT) and 4-hydroxyindole-3-acetic acid (4HIAA)” [43]. Grieshaber showed that psilocin undergoes conjugation with glucuronic acid in the liver and it can be found in the urine as the psilocin-glucuronide conjugate [43]. Psilocin is a partial 5HT2a agonist [14], but it is also agonistic at other serotonin receptors, with little dopaminergic or noradrenergic action: these activities explain the onset of sympathetic stimulations, such as tachycardia and hypertension, along with those of the predominant hallucinogenic effects [44]. Psilocybin is orally active, with effective doses in the range of 6–20 mg. The onset of action is typically 20–40 min, peaked after another 60–90 min, lasting for another 60–120 min [45].
The synthetic 4-substituted tryptamines include 4-hydroxy-N,N-diethyltryptamine (4-HO-DET), 4-hydroxy-N,N-diisopropyltryptamine (4-HO-DiPT), 4-hydroxy-N-isopropyl,N-methyltryptamine (4-HO-MiPT) and their acetic acid derivatives (for example, 4-acetoxy-N,N-diethyltryptamine and 4-acetoxy-N,N-diisopropyltrypt-amine): all these compounds, according to users [46], seem to have similar actions to psilocin, but very little scientific information about their properties is available.
Go to:
5 POSITION MODIFIED TRYPTAMINES
Bufotenine (Fig.
5
5), or 5-OH-dimethyltryptamine (5-OH-DMT), is an N-alkylated derivative of serotonin and it was first synthesized by Wieland in 1934 [47]: it can be isolated from plants Piptadenia peregrina and P. macrocarpa (Mimosaceae), the mushrooms Amanita (spp) and from the skin glands of toads (Bufo spp.) [48]. Fuller, studying the metabolism, the tissue distribution and the effects of 5-OH-DMT on rats, showed that, after injection in the animals, it was present in highest concentration in lung and in heart rather than in brain: this, probably, means that it does not cross well the blood-brain barrier, just as serotonin [49]. In tissues studies, he found that bufotenine was rapidly eliminated, disappearing within 8 hours, after reaching peak concentrations at 1 hour. Moreover, the large increases of 5-hydroxyindoleacetic acid (5HIAA), the endogenous metabolite of serotonin, in all tissues suggested that bufotenine was metabolized by MAO-A through the pathway of oxidative deamination.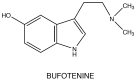
| Fig. (5) |
Intranasal administration (nine bioassays from 5 to 100 mg): he reported the visionary threshold at 40 mg; the range from 5 to 30 mg caused the onset of psychoactive effects at all doses, “with closed-eye luminosity and scintillation commencing at 20 mg”. He recorded that at 5 minutes after the 40 mg administration, he felt the onset of tinnitus; at 25 minutes he felt the body effects typical of the intakes of tryptamines; the peak arrived between 35 and 40 minutes and all the psychoactive sensations vanished after 90 minutes. He noted also that he did not feel discomfort or side-effect.
Sublingual administration (50 mg): he reported that “free base bufotenin taken sublingually was found to be similar to intranasal use”, for what concerned “the potency, duration, and psychedelic action”.
Intrarectal administration (30 mg): he reported mild physical effects that developed quickly and that lasted roughly an hour. He felt psychotropic effects, like scintillations with closed eyes, only when he co-administered harmaline with bufotenine suppository.
Administration via inhaling vapor (2-8 mg): he inhaled 2, 4, 6 and 8 mg of bufotenine. All doses were decidedly psychoactives, increasing in potency proportionally to dosage. The peaks were attained after four to five minutes with a gradual diminution of the effects for an hour.
Oral administration (100 mg): he reported that 100 mg dose was most decidedly psychoactive. Tinnitus manifested at 20 minutes, the peak was reached at one hour and 30 minutes “with all the classic tryptaminic bodily sensations and mild psychoptic effects, but absent colored patterns” [50].
The 5-substituted tryptamines, also include 5-methoxy-α-methyltryptamine (5-MeO-AMT), 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT), 5-methoxy-N,N-diisopropyltryptamine (5-MeO-DiPT, “Foxy Methoxy”), 5-methoxy-N,N-methylisopropyltryptamine (5-MeO-MiPT): these compounds inhibit monoamine re-uptake, but they have few effects on monoamine release.
5-methoxy-α-methyltryptamine (5-MeO-AMT) is a psychedelic tryptamine which presents structural similarities to the amphetamines: because of these characteristics, 5-MeO-AMT was occasionally sold under the guise of LSD, as emerges from the reports of Drug Enforcement Agency (DEA) about seizures of sugar cube, or blotters LSD-style, containing this tryptamine [51]. Its mechanism of action is characterized by binding to 5-HT1A and 5-HT2A receptors [52, 53] it also inhibits the monoamines reuptake and increases their release in brain synaptosomes at micromolar concentrations [16]. 5-MeO-AMT is orally administered in a range of 2.5-4.5 mg and the duration of the effects reported is about 12-18 hours. If misrepresented as LSD, 5-MeO-AMT can be very harmful or even fatal: in fact, incorrect or excessive administration of the substance, mistaken for LSD, has been the cause of several hospital admissions [54] or sudden deaths [55].
It is likely that the danger related to 5-MeO-AMT assumption is due to its sympathomimetic effects, that include, as side effects, cardiac arrhythmia and seizure.
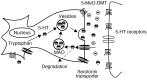
5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) is a naturally occurring psychoactive indolealkylamine substance, closely related to DMT and bufotenine. It was initially isolated from the bark of Dictyoloma incanescens [56], but it was found also in the venom of Colorado River Bufo alvarius, secreted by parotoid and tibial glands. This compound is a potent, fast acting hallucinogen [57], producing psychedelic effects, with a short duration in humans: it has high affinity for the 5-HT1A serotonin receptor, causing many physiological and behavioral changes. It can be administered by different routes, as reported in the Table
1
1.

| Table 1 |
For this reason, to produce a prolonged hallucinogen effect when orally administered, 5-MeO-DMT requires the concomitant use of a MAOI, such as harmaline. This co-administration could lead to an hyperserotonergic syndrome because of the agonistic activity of these two substances on the serotoninergic system: in fact, MAOI activity causes an increasing exposure to the parent drug (5-MeO-DMT) and to the active metabolite (bufotenine), originating a possible fatal toxicity (Fig.
6
6).
| Fig. (6) |
5-methoxy-diisopropyltryptamine (5-MeO-DiPT), or “Foxy Methoxy”, produced by the addition of two isopropyl groups to the terminal nitrogen of the aminoethyl group, and methoxylation of the 5-position of the indole ring, is a synthetic designer drug which was first synthesized and described by Shulgin.
Sogawa “investigated the actions of 5-MeO-DiPT against monoamine neurotransmitter transporters, using COS-7 cells heterologously expressing these transporters and rat brain synaptosomes”, and demonstrated that “5-MeO-DiPT acts as a competitive serotonin transporter (SERT) inhibitor and has an inability to cause reverse transport, underlying its serotonergic actions” [59]. Agonistic activity at serotonin receptors could lead to permanent damages to serotonergic neurons with neuropsychiatric implications [60]. Fantegrossi reported also that “5-MeO-DiPT had affinity for receptors relevant to hallucinogenic effects” observed in vivo, and that the psychotropic activity of 5-MeO-DiPT might therefore be caused by its association with post-synaptic 5-HT receptors [61]. In human biological samples, such as blood and urine, 5-MeO-DiPT is mainly metabolized in 5-hydroxy-N,N-diisopropyltryptamine (5-OH-DiPT) and in 5-methoxy-N-isopropyltryptamine (5-MeO-NiPT) [62]. 5-MeO-DiPT is orally active, and dosages between 6–12 mg are reported by Shulgin, but it can be also smoked, or snorted. Its onset of action is in 20 to 30 minutes, and its effects last from 3 to 6 hours. The users experienced euphoria, disinhibition, increased sociability, visual and auditory hallucinations, feeling of love but also less desiderable effects like myoclonus, restlessness, insomnia and anxiety, as well as emotional distress like nausea, vomiting and diarrhea.
5-methoxy-N,N-diallyltryptamine (5-MeO-DALT) was first synthesized by Shulgin and although information on this tryptamine was available since 2004, “its use as hallucinogenic drug has been reported only occasionally in on-line fora” [10]. According to the European Database on New Drugs (EDND), it was first sequestered as two grey tablets, in 2006 at Helsinki airport [10]. There is little scientific literature on this tryptamine: Nonaka showed that 5-MeO-DALT seemed to support G protein activation via serotonin 5-HT1 receptor with experiments on rat brains [63]. Moreover, it did not show effect on dopamine, serotonin and norepinephrine re-uptake and presented a slight monoamine-releasing activity [16]. 5-MeO-DALT is mainly found in powder form that is taken orally or nasally. The range of doses and effects is unusually broad. Shulgin reported dosages for oral administration from 12 to 25 mg: users reported that the onset of its effects was within 15 minutes, the peak was reached in 30 minutes and the main effects lasted for 2-4 hours, including euphoria, visual hallucinations, walking difficulties, and ‘out of body’ experiences.
5-methoxy-N-methyl-N-isopropyltryptamine (5-MeO-MiPT) is an analogue of the more popular drug 5-MeO-DiPT (“Foxy”) and, for this reason, it is nicknamed "Moxy".
With regard to its mechanism of action, Fantegrossi demonstrated that 5-MeO-MiPT, as the 5-MeO-DiPT, suppressed the re-uptake of 5-HT and the re-uptake of dopamine and norepinephrine. It showed little dopamine, 5-HT or norepinephrine releasing activity [61].
5-MeO-MiPT can be smoked or orally administered. The dosage for oral use is from 4 to 6 mg and the duration of the effects is from 4 to 6 hours, while if it is smoked the dosage is 12-20 mg. The onset of the psychoactive sensations, after oral assumption, is from 15 to 20 minutes, the peak is reached from 45 to 60 minutes and the return to normality is registered after about 10 hours. If it is smoked, the onset is immediate, the duration of the effects is from 2 to 5 hours and symptoms disappear after about 2-4 hours. The effects reported by the users including euphoria, increased tactile sensations, relaxation, visual distortions and difficulty in sleeping [64].
Go to:
ERGOLINES: HAWAIIAN BABY WOODROSE AND MORNING GLORY SEEDS
Ergine, or lysergic acid amide (LSA), is an alkaloid of the ergoline family closely related to LSD, found in the seeds of Argyreia nervosa (Hawaiian baby woodrose) and Ipomoea violacea (Morning Glories).
Hallucinogenic activity of LSA occurs with 4-10 seeds of Argyreia nervosa or with 150–200 seeds (3–6 g) of Ipomoea violacea: seeds could be crushed or eaten whole, or also drunk as an extract, after soaking in water [42] The onset of the hallucinatory effects, after ingestion of Hawaiian Baby Woodrose, is from 20 to 40 minutes and their total duration is from 5 to 8 hours: the plateau is reached after 4-6 hours and the return to normality is after 1-2 hours from the plateau. The principal sensations reported by the users include relaxations, mild euphoria, feelings similar to alcohol intoxication [65], psychedelic visual effects such as enhanced colors, but also anxiety, nausea, confusion and paranoia [66]. However, as regards to the assumption of the Morning Glory seeds, the onset of the hallucinatory effects is from 30 to 180 minutes and they last for 4 to 10 hours. The users reported that they return to normality after about 24 hours [67].
Morning Glory seeds assumption can produce heightened sense of awareness of colors, textures and sounds: the users reported also a diminished sense of reality, an increased sense of suggestibility, but also a sense of anxiety and panic, nausea, vomiting, and psychotic episodes [68].
Go to:
EFFECTS DUE TO ASSUMPTION OF TRYPTAMINES REPORTED ON INTERNET
The information regarding the effects of tryptamines reported in this paragraph, is collected from websites, so these effects are exclusively the result of personal experiences of authors and do not follow the use of the scientific method. They are reported because Internet is probably one of the most effective sources of timely information for any specialist or organization interested in the subject.
The tryptamines users are not always aware of what they have purchased and of its chemical constituents, and there are many risks associated with the possibility of overdose, repeated administration and incorrect route of administration. In this section, we are going to report the main effects connected with the use of tryptamines, included the one reported as “positive” by authors.
The routes of administration of the various tryptamines are one of the key factors that need to be evaluated: some can be taken orally or intramuscularly or intravenously, or can be smoked or inhaled, showing an increased of hallucinogenic sensations, especially in relation to dose and repeated-dose toxicity due to a delayed onset of phenomenology.
Go to:
ASSUMPTION OF RING UNSUBSTITUTED COMPOUNDS
Ring unsubstituted compounds such as α-methyltryptamine (AMT) and α-ethyltryptamine (AET) have stimulant properties as well as hallucinatory effects [18]. AET is described as having psychoactive properties similar to MDMA. Tables
2
2 and
3
3 summarize AMT and AET experiences as reported on http://www.erowid.org [69, 70].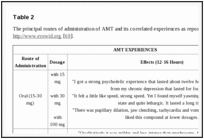
| Table 2 |

| Table 3 |
4
4 are summarized DMT experiences in relationship with the route of administration reported on the website http://www.erowid.org [71].
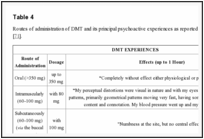
| Table 4 |
Unlike DMT, other unsubstituted tryptamines are orally active. Smoking and nasal insufflations are also common methods of administration. Visual hallucinations are universal reported as “positive” effects by users [74]. General effects reported as “positive” on www.erowid.org by users of unsubstituted tryptamines, that are active after ingestion, include: ‘rushing’ sensation, both opened and closed eyes ‘pleasant’ visual hallucinations, increased mood, energy, libido, concentration and empathogenic qualities [75]. These effects are collected in Table
5
5.
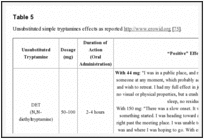
| Table 5 |
Unsubstituted simple tryptamines effects as reported http://www.erowid.org [75].Go to:
ASSUMPTION OF 4-POSITION MODIFIED TRYPTAMINES
Synthetic 4-substituted tryptamines are orally active and they seem to produce similar effects to those mediated by psilocin. General effects include: increased laughing, intense visual hallucinations, ‘rushing’ sensation, euphoria, increased libido, enhanced tactile sensations, increased concentration and a feeling of warmth and inner peace [76]. Dosage and effects of most common synthetic 4-substituted tryptamines on www.erowid.org, are reported in Table
6
6 [75].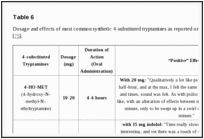
| Table 6 |
Dosage and effects of most common synthetic 4-substituted tryptamines as reported on http://www.erowid.org [75].Go to:
ASSUMPTION OF 5-POSITION MODIFIED TRYPTAMINES
The 5-substituted tryptamines are more powerful than the unsubstituted molecules, but clinical effects reported are similar among them. Effects reported as “positive” by users of 5-substituted tryptamines include [58]: euphoria, an increasing of energy, libido, concentration and sociability, and a reduction in fear and anxiety [57]. Table
7
7 represents a summary of the reported effects of the personal experiences of the users of some 5-substituted simple tryptamines by Alexander and Ann Shulgin [75].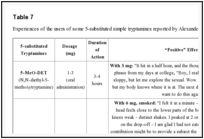
| Table 7 |
8
8 are summarized the main adverse effects reported by users in relationship with the tryptamine derivatives assumed.
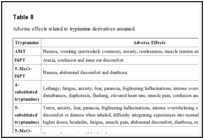
| Table 8 |
Adverse effects related to tryptamine derivatives assumed.Go to:
TRYPTAMINES EFFECTS AND INTOXICATIONS: CASE REPORTS FROM EMERGENCY ROOMS AND CORONERS’ ACTIVITIES
Gathering data on the use of these new psychoactive substances it is very difficult, since they are not collected in a systematic way and often these information come from unverified sources (drug-related fora or websites). So the only evidences that have an epidemiological significance derive from arrests and seizures by law enforcement agencies [14], from the numbers of enquires to poisons centers, from the reports of the emergency departments or from admissions to hospital. Unfortunately, the limited number of these official reports may underestimate the true extent of the problem [80]: statistics on deaths may also be available, but often these publications do not provide information on toxicity or mode of action of these substances as completely lacks the analytical confirmation of the agents involved, and the causes of death are ascertained or through the testimonies of family members and friends, or after the discovery of the substance at home or in the clothes of the deceased. Nevertheless, in scientific literature are reported a consistent number of cases of acute poisoning due to the increasingly widespread use of these new psychoactive substances: many of these are the result of emergency room experience. As the amount of tryptamines necessary to cause psychotropic effects is minimal, and the interval of occurrence of effects is rather delayed [39], there is a risk of inappropriate new assumptions, that could lead to poisoning or overdose.
Holstege reported the case of poisoning of a college student (male, 21 years old) came in E.D. after ingestion of 270 mg of AMT purchased on the internet [81]. The subject reported that he had used the same AMT lot of different times for its psychedelic properties, and to have miscalculated the last dose, ingesting a dose about 10 times higher than the previous. After an hour of ingestion presented hypertension (BP 183/93 mmHg, heart rate of 52 bpm), with normal respiratory rate and apyrexia. The patient was awake, hyper-alert, oriented with mydriasis (10 mm diameter), moderate tremor, delay in response times, restless, in exaggerated startle reaction and visual hallucinations. After 10 hours the symptoms begun to resolve. The diagnostic tests were normal, except for a slight value hypokalemia. He was discharged without complications.
Long reported during the EAPCCT XXIII International Congress the case of a patient (male, 17 years) arrived at the hospital with sympathomimetic effects after ingestion of AMT. The subject was found almost completely naked, while ran, and cried, and did not have previous medical or psychiatric history. He presented tachycardia (160 beats/min), extreme sweating (with temperature 37.28°C), and mydriatic pupils of 6-7 mm and reactive. The rest of the physical examination appeared normal, and the patient was immediately sedated with 6 mg lorazepam administrating by intramuscular route. Routine laboratory tests were normal, as well as the analysis of the cerebrospinal fluid and brain scan. Analysis of urine were negative for cannabinoids, cocaine, amphetamines, and phencyclidine. The day after the subject indicated the assumption of AMT, purchased on the Internet with the instruction for insufflation of 100 mg. The patient, however, reported that he had ingested the powder rather than take it by inhalation, with onset of symptoms after 15 min. HPLC analysis of the urine has confirmed the presence of the AMT and the absence of cocaine, phencyclidine, and amphetamines. The authors conclude that the intake of AMT involves rapid heartbeat, sweating, agitation and hallucinations and suggest supportive therapy, including sedation with benzodiazepines [82].
A case of intoxication after ingesting an unknown amount of DPT is reported in a 19 years old female by Dailey: when she arrived in E.D. presented hallucinations, extreme agitation and tachycardia (200 bpm). Clinicians administered 3 mg of lorazepam to resolve the state of agitation. Toxicological analysis did not reveal positivity for DPT: the assumption of DPT was ascertained only for the presence with the patient, of a vial with a label bearing the inscription “for research purposes only” [83].
Brush, reported a case of an adolescent (17 years old male) whose use of the Internet to obtain drug information led to severe poisoning from the combination of a monoamine oxidase inhibitor, harmaline, and a hallucinogenic tryptamine, 5-methoxydimethyltryptamine (5-MeO-DMT) [84]. He purchased on the Internet some Syrian rue seeds containing harmaline, a natural MAOI [76]. After ingestion of seeds, smoking 10 mg of 5-MeO-DMT, and insufflation of further 15-20 mg, his friends found him collapsed, agitated and hallucinating. He arrived in E.D. with tachycardia (heart rate 186 bpm) and hyperpyrexia (40.7°C). He required physical restraint and clinicians administered to him 2.5 mg of IV lorazepam. This case illustrates the extreme danger of simultaneous administration of tryptamine with MAOIs: indeed, the concomitant use of harmaline and 5-MeO-DMT reduces deamination metabolism of the parent drug, leading to a prolonged and increased exposure to 5-MeO-DMT, as well as to its active metabolite bufotenine [57].
Wilson, reported the case of a 23-year-old man arrived to the Emergency Department after ingestion of a 5-MeO-DiPT homemade capsule. He did not lament visual nor auditory hallucinations, but only sensory distortion such as formication. Paranoia symptoms were observed. The patient was put under observation and then discharged. Toxicological analysis performed in blood and urine with gas chromatography–mass spectrometry revealed the presence of “Foxy” (5-MeO-DiPT) in the following concentration 0.14 (serum) and 1.6 μg/mL (urine), and its metabolite 5-methoxy-indole acetic acid was found in urine 0.17 μg/mL [85].
The ingestion of “Foxy” was associated with rhabdomyolysis and transient acute renal failure: Alatrash reported the ingestion of 25 mg of 5-MeO-DiPT in a healthy 23 years old man who arrived in E.D. presenting an altered state of consciousness characterized by hallucinations, tachycardia, hypertension and hyperpyrexia. Clinical investigations revealed renal impairment, metabolic acidosis and rhabdomyolysis. Toxicological analysis performed on a urine sample was negative for amphetamines, cannabinoids, cocaine, ethanol and barbiturates: the tests, executed in E.D., didn’t confirm the presence of 5-MeO-DiPT [86].
The ingestion of an unusual dose of “Foxy” in a 19 years old man is reported by Smolinske. On arrival, he had hallucinations, hypertension, tachycardia, mydriasis, and catalepsy. Laboratory analysis revealed hyperglycemia, glycosuria and an increased white cell count. A urine drug screening resulted positive for cocaine and phencyclidine: this test didn’t reveal the presence of 5-MeO-DiPT. Symptoms were resolved within two hours after administration of lorazepam [87].
Meatherall described a 21-year-old man who arrived to the Emergency Department about 1.5 h after consuming a capsule to get high, named “Foxy”, and sold to him on the street: he presented no nausea, pain, or visual deficit, he was alert and oriented, the only relevant clinical observation was his inability to move his limbs. He denied taking any other recreational drug or alcohol. He had a blood pressure of 122/56 mm Hg, with 106 pulses and a respiration rate of 20. His pupils were equal and reactive with intact extra ocular movements and there was no motor sensory deficit. His cardiorespiratory examination was unremarkable. The patient received medical support until the symptoms did not disappear, about 3.5 hours after ingestion, then he was discharged from hospital. The toxicological analysis performed on the urine sample did not show presence of alcohol, but the immunoassay screening test was positive to cannabinoids and opiates: these results were, then, confirmed by GC/MS analysis. Acetaminophen and caffeine were detected in the acid-neutral drug screen performed with GC/MS. The GC/MS basic drug screen, indeed, revealed a large 5-MeO-DiPT peak, codeine, nicotine, cotinine, acetaminophen, caffeine, and two other peaks that were assumed to be 5-MeO-DiPT metabolites. The quantification of 5-MeO-DiPT was performed by GC/MS resulting at a concentration of 1.7 μg/mL in the urine sample [88].
The review of the American Association of Poison Control Centers’ Total Exposure Surveillance System (AAPCC TESS) database found 41 exposures to “Foxy” between April, 2002 and June, 2003, resulting in moderate to severe toxicity in 68% of these cases. Clinical effects commonly involved agitation (59%), hallucinations (39%), tachycardia (37%), hypertension (17%), and confusion (15%). Tremors and seizures were rare [89].
Shimizu described a case of intoxication in a 27-year-old Japanese businessman after a single ingestion of the mixture of methylone and 5-MeO-MiPT: he had no previous psychiatric or medical illness and he had never used recreational drugs as methamphetamine, MDMA or organic solvents. The patient referred only sleep irregularities: he reported that, to solve his sleep disturbance, decided to take a drug without professional consultation. Although he did not know in depth the chemical and physical characteristics of methylone, he believed it could help feel him free from insomnia. Then, he bought on the Internet, the drug 1 g as pure methylone powder: but, toxicological analysis performed on the substance indicated that drug was composed of about 60% methylone (120 mg) and 38% 5-MeO-MiPT (76 mg). He took approximately 200 mg of the drug powder by oral administration and thirty minutes later, he started to feel nausea and sick in the stomach. When he arrived in hospital, he presented psychomotor excitement and he was shouting without apparent reason. He showed dilated pupils and sweating, pyrexia (37.8 °C), tachycardia (150 bpm) and his blood pressure was 144/81 mm Hg. After a gastric lavage a rapid drug screening device was used to examine a gastric fluid sample and urine: the assay systems showed no immuno-reactivity for the most common drugs of abuse (phencyclidine, benzodiazepines, cocaine metabolites, amphetamine and methamphetamine, cannabinoids, opiates, barbiturates, and tricyclic antidepressants). He still exhibited symptoms of substance-induced psychomotor excitement, more than 3.5 h from drug administration. He was observed and received medical assistance until the following day, then discharged without any known sequel: however, he did not remember anything of what happened during acute intoxication [90].
Jovel described a case involving a 20-year-old college student brought to the E.D. after the ingestion of a dietary supplement capsule named “Lucy-N-Nate” that contained the novel synthetic tryptamine 5-MeO-DALT in unknown concentration. Although denying any other illicit assumption, his urine toxicological screening resulted positive for cannabinoids and amphetamines. At the admission he displayed diaphoresis, agitation, combativeness, flushes and warm sensation. Heart beat rate showed marked tachycardia (180–200 bpm) and tachypnea was observed. Benzodiazepines and haloperidol were administered in order to try to manage the patient’s severe agitation with few results, therefore clinicians decided to proceed to deeper sedation and intubation. Rhabdomyolisis and acute renal failure were observed but resolved with supportive measures and the patient after three days was transferred to the inpatient psychiatric unit, and the following day he was discharged without symptoms or psychiatric sequelae [91].
Go to:
FATALITIES INVOLVING TRYPTAMINE MISUSE
The use of tryptamines with recreational purposes may be associated with a higher risk of overdose, or due to a wrong dosage, as the information on these compounds available on the Internet are exclusively based on the first-hand personal accounts presented in discussion fora, or to a repeated administrations, because often the onset of the desired effects can occur at a distance of several hours from the assumption.
In February 2003, the Miami-Dade County Medical Examiner Department reported the first known death in the country related to the assumption of α-methyltryptamine (AMT). The case in Miami, involved a 22 years old college student who found deceased, about 12 hours after he confided to his roommate he was "taking hallucinating drugs" and he was able to "discover the secret of the universe". The roommate reported that he was shaking and sweating profusely, waving a knife, and threatening to commit suicide. The roommate tied him to the bed for his own protection and left him to "sleep it off". Approximately 12 h later, the roommate discovered the man lying in bed unresponsive. From the scene was recovered an empty 1-g vial of AMT. Immunoassay analysis of urine and gastric contents performed with enzyme immunoassay technique were positive for amphetamines and the basic drug blood screen performed with GC/MS detected a small peak, later identified as AMT. Volatile substances were screened using headspace gas chromatography (GC/HS). Postmortem toxicological analysis performed on iliac vein blood, gastric contents, liver and brain revealed the presence of AMT in the following concentrations (Table
9
9) [92, 48].
| Table 9 |
Morano reported a case of a 19 years old female who ingested a glass of beer containing two ‘hits’ of white powder that she described as MDMA. After ingestion she became confused, vomited and had a cardiac arrest. Autopsy revealed bilateral “pulmonary edema and generalized visceral congestion with some epicardial petechiae”. Toxicological findings showed the following AET tissue distribution: blood (heart) 5.6 mg/L, urine 80.4 mg/L, vitreous 2.4 mg/L, bile 22.0 mg/L, stomach contents 52.9 mg.; in liver, kidney and brain AET was found in a concentration of 18.3 mg/g, 24.0 mg/g and 16.2 mg/g respectively. MDMA was no revealed in autoptical samples [94].
Another case of fatal intoxication with AET is reported by Daldrup. After ingestion of α-ethyltryptamine a young man developed agitation, hyperpyrexia: he showed effects similar to those known from intoxication with amphetamines, MAO inhibitors, and thymoleptics. The exact amount of α-ethyltryptamine taken are not known, but it could have been in the range of 700 mg. The level in postmortem blood was 1.1 mg/l. Malignant hyperthermia is discussed as a possible cause of death [95].
In 2005, a 25 years old white male was found dead in a national park, where he was camping with his family, the morning after consuming a herbal hallucinogenic extract containing β-carbolines and hallucinogenic tryptamines [96]. An autopsy was performed the day after the body was discovered and no anatomic cause of death was found. “External examination identified only the presence of lividity that was fixed on the posterior surface of the body, except in areas exposed to pressure. Internal examinations, both gross and microscopic, were unremarkable except for some tissue congestion and edema”. Toxicological analysis were performed on specimens (central and peripheral blood, urine, gastric contents, bile, kidney, brain, and liver) collected at autopsy. The heart blood and urine samples were tested for volatile substances (methanol, ethanol, acetone and isopropanol by headspace gas chromatography) and therapeutic and abused drugs: no ethanol or other volatile substances were detected. Diphenhydramine was detected exclusively in the urine sample. “An unidentified peak was detected in both the blood and urine specimen on the alkaline drug screen: subsequent mass spectral analysis identified the substance as 5-MeO-DMT”. In the heart blood sample was identified N,N-dimethyltryptamine (0.02 mg/L), 5-methoxy N,N-dimethyltryptamine (1.88 mg/L), tetrahydroharmine (0.38 mg/L), harmaline (0.07 mg/L), and harmine (0.17 mg/L). Tetrahydroharmine (THH) “was present in higher amounts than both harmaline and harmine in all samples with the exception of the gastric contents, where the amount of harmine was 10-fold greater than that of THH”. The medical examiner ruled that the cause of death in this case was hallucinogenic amine intoxication.
Tanaka reported a fatal case of intoxication with “Foxy” (5-methoxy-N,N-diisopropyltryptamine). The decedent (male A) was a 29-year-old. The male A was a sexual partner of male B, who injected an aqueous solution of 5-MeO-DiPT into the anus of male A using a dropper, with the aim of enhancing sexual pleasure. Following the rectal administration, male A showed adverse effects including abdominal symptoms and intense agitation, so he was rushed in hospital, but he died on the following day. “Autopsy findings revealed periarteritis nodosa, involving the heart and liver, an area of myocardial ischaemia, leukocytosis, advanced pulmonary congestion and pulmonary alveolar haemorrhage and periprostatic bleeding. Blood and urine specimens were submitted for toxicological examination”: 5-MeO-DiPT and its two metabolites, 5-hydroxy-N,N-diisopropyltryptamine (5-OH-DIPT) and 5-methoxy-N-isopropyltryptamine (5-MeO-NIPT), were identified by LC–MS. The level of 5-MeO-DIPT, 5-OH-DIPT and 5-MeO-NIPT in blood was 0.412, 0.327 and 0.020 µg/ml, while their concentrations in urine sample were 1.67, 27.0 and 0.32 µg/ml, respectively. These blood and urine levels were higher than published data for such poisoning. “In addition, no ethanol, therapeutic and abused drugs were detected in serum and urine specimens in this case. Based on the autopsy and toxicological findings, the cause of death was acute cardiac failure due to neuro-toxocity resulting from an overdose of 5-MeO-DiPT ” [62]. Unconfirmed reports on the Erowid website hypothesized that a 100 mg dose was used rectally, while a normal dose would be 10 mg [97].
In some cases the death of users of tryptamines for recreational purposes, was not caused by overdose or incorrect dosages, but by changes in the state of consciousness that led to the outbreak of irrational behaviors, extremely dangerous.
A 26 years old male was killed after walking onto the slow lane of a motorway and being hit by a lorry. He was seen to walk in front of a heavy goods vehicle while he was grinning. “At the inquest it was reported that he had snorted 350 mg of 5-MeO-DALT” [98] “(when the normal dose is 25 mg) in a public house with a friend who bought 1 g of it over the Internet”. During the autopsy, the coroner found a significant head injury with a base of skull fracture, cerebral and pulmonary contusions. The pathologist recorded the cause of death as “fractured base of skull” [10].
An initial toxicological investigation found that the postmortem femoral blood contained an atracurium breakdown product (laudanosine) and propofol, which presence is consistent with medical intervention. The blood alcohol concentration was 22 mg/dL. “A compound was detected in blood with a distinctive tryptamine-like UV spectrum, but tryptamine itself is also regularly observed as a putrefactive compound. No other drugs were detected”. Following “further information about the deceased's consumption of 5-MeO-DALT, supplementary toxicological analysis of the post mortem femoral blood samples were undertaken, using liquid chromatography with mass spectrometry detection (LC–MS), as it can be difficult to distinguish between tryptamine and other naturally occurring tryptamine-related compounds and those with methoxytryptamine derivatives (including 5-MeO-DALT) with similar UV spectra”. LC–MS analysis indicated that the compound was 5-MeO-DALT, but there are no information about its quantification.
It was reported another case of death [99] of a man who, after ingesting Hawaiian baby woodrose seeds [100], jumped from a building: ergine was found in his post mortem blood and urine [11].
Go to:
ANALYTICAL METHODS FOR TRYPTAMINES
Tryptamines are not routinary detected with common immunoassay-based techniques, leading, for example, to misunderstanding in diagnosis or therapy in Emergency Departments, as toxicological screening tests could result completely negative.
Extensive laboratory analysis is required for the identification of these compounds, involving the use of techniques with high selectivity and sensitivity such as mass spectrometry (MS). But, even using powerful instrumentations, the new substances could be missed, either because, working in a full-scan mode, reference mass spectra are not yet included in libraries, or because the fragments generated during the analysis in selected ion monitoring (SIM) show nondescript and not monitored fragmentation patterns [76].
Moreover, instrumental screening methods were developed also for other NPS, but they do not always include tryptamines [101-103].
Several analytical approaches for tryptamines were published in scientific literature since 2003. Both screening methods [104-107] and confirmatory methods were developed for the detection of tryptamine derivatives which had clinical and forensic relevance [62, 85, 88, 90, 92, 96, 105, 106, 108-111], using the hyphenated chromatography (GC and LC) mass spectrometry approach.
Whole blood, serum, plasma and urine were used for clinical studies [85, 88, 90, 104, 110, 111], while several biological matrices were investigated for forensic purposes [62, 92, 96].
Different extraction methods and sample preparation procedures were proposed: liquid liquid extraction (LLE) [62, 85, 88, 96, 112], solid-phase extraction (SPE) [92, 104, 105, 108, 109, 111], dilution in solvent [107, 110, 111, 113, 114] and protein precipitation (PP) [106].
The separation techniques used were gas chromatography (GC) and High Performance Liquid Chromatography (HPLC): in some recent papers, Ultra High Performance Liquid Cromatography (UHPLC) was used providing a more efficient analysis in a shorter time [106, 111]. The separation techniques were coupled with mass spectrometers, mainly for forensic purposes.
Several analyzers were used: (1) a single quadrupole in full-scan mode [90, 107, 112-114] and in SIM mode [62, 92, 96, 104, 108, 109]; (2) a quadrupole ion trap in full-scan mode [88]; (3) a triple quadrupole in the following analyzer mode: MRM, SRM, PIS, IDA and in full-scan mode [105, 110-112]; (4) a high resolution Orbitrap [114]. It was also used a TOF analyzer with MALDI ionization system, in order to compare the fragmentation pattern with that obtained with other analyzers [107].
Results of analysis of substances seized from law enforcement agencies were published too [90, 107, 113, 114], resulting in a useful source of information for specialists, including the personnel working in Emergency Departments. When NPS are found, if a reference standard is not available, NMR is needed for structural elucidation. Infrared (IR) spectroscopy is another powerful tool for bulk analysis. Hugel reported both the IR spectra and MS spectra of N,N-dimethyltryptamine, N,N- diethyltryptamine, N,N-dipropyltryptamine, and 5-methoxy-N,N- diisopropyltrypt-amine [115].
Key information of selected analytical methods reported in scientific literature is summarized in Table
10
10.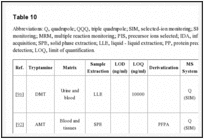
| Table 10 |
Abbreviations: Q, quadrupole; QQQ, triple quadrupole; SIM, selected-ion monitoring; SRM, selected reaction monitoring; MRM, multiple reaction monitoring; PIS, precursor ions selected; IDA, information-independent acquisition; SPE, solid phase extraction; ...Go to:
CONCLUSIONS
Many psychoactive substances belong to the chemical class of tryptamines. There are old compounds known for their hallucinogenic properties, such as psilocybin in ‘Magic mushrooms’ and dimethyltryptamine (DMT) in Ayahuasca brews, and there are several New psychoactive substances (NPS), emerging drugs whose chemical structures are similar to other psychoactive compounds but not identical, so that they represent a “legal” alternative to internationally controlled drugs. The comprehensive review of the published literature carried out permitted to find not only many scientific publications but also useful information from non peer-reviewed sources, including media reports and Internet resources such as drug user web fora. It was possible to collect and organize information on chemical characterization, pharmacology and toxicity of these drugs, according to a proposed classification based on their chemical structures. The articles, reporting cases of death related to intake of these substances, showed the need of more effective policies to promote timely exchange of information. Internet is “a growing source of on-line drug trafficking”: products purchased on Internet may vary over time proposing chemicals always different, not listed in literature, meaning that users are often unaware of what or how much they are taking, causing increasing risks to public health. For this reason information from Internet, together with results of analysis of substances seized from law enforcement agencies and reports of cases of death related to intake of these substances, organized in the way we proposed in this review, provides an effective tool for specialists facing this emerging threat to public health and public security, not only forensic scientists but also the personnel working in Emergency Departments.
Go to:
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
Go to:
ACKNOWLEDGEMENTS
Declared none.
Go to:
References
1. Available from http://www.unodc.org/documents/scientific/NPS_2013_SMART.pdf . 2014. [Last access on 24th August].
2. Zuba D. Identification of cathinones and other active components of “legal highs” by mass spectrometric methods. TrAC. 2012;32:15–30. doi: 10.1016/j.trac.2011.09.009. [Cross Ref]
3. Musselman M.E, Hampton J.P. “Not for human consumption”: a review of emerging designer drugs. Pharmacotherapy. 2014;34(7):745–757. doi: 10.1002/phar.1424. [PubMed] [Cross Ref]
4. Davies S, Wood D.M, Smith G, Button J, Ramsey J, Archer R, Holt D.W, Dargan P.I. Purchasing ‘legal highs’ on the Internet--is there consistency in what you get? QJM. 2010;103(7):489–493. doi: 10.1093/qjmed/hcq056. [PubMed] [Cross Ref]
5. Arnold C. The new danger of synthetic drugs. Lancet. 2013;382(9886):15–16. doi: 10.1016/S0140-6736(13)61512-3. [PubMed] [Cross Ref]
6. Available from https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/119139/acmdnps2011.pdf . 2014. [Last access on 24th July].
7. Brandt S.D, Freeman S, McGagh P, Abdul-Halim N, Alder J.F. An analytical perspective on favoured synthetic routes to the psychoactive tryptamines. J. Pharm. Biomed. Anal. 2004;36(4):675–691. doi: 10.1016/j.jpba.2004.08.022. [PubMed] [Cross Ref]
8. Martins P.B, Freeman S, Alder J.F, Passie T, Brandt S.D. Profiling psychoactive tryptamine-drug synthesis by focusing on detection using mass spectrometry. TrAC. 2010;29(4):285–296. doi: 10.1016/j.trac.2010.01.009. [Cross Ref]
9. Jones R.S. Tryptamine: a neuromodulator or neurotransmitter in mammalian brain? Prog. Neurobiol. 1982;19(1-2):117–139. doi: 10.1016/0301-0082(82)90023-5. [PubMed] [Cross Ref]
10. Corkery J.M, Durkin E, Elliott S, Schifano F, Ghodse A.H. The recreational tryptamine 5-MeO-DALT (N,N-diallyl-5-methoxytryptamine): a brief review. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;39(2):259–262. doi: 10.1016/j.pnpbp.2012.05.022. [PubMed] [Cross Ref]
11. Gibbons S. ‘Legal Highs’ - Novel and emerging psychoactive drugs a chemical overview for the toxicologist. Clin. Toxicol. 2012;50(1):15–24. [PubMed]
12. Chilton WS, Bigwood J, Jensen RE. Psilocin, bufotenine and serotonin: historical and biosynthetic observations. J. Psychedelic Drugs. 1979;1(1-2):61–69. [PubMed]
13. Lyttle T, Goldstein D, Gartz J. Bufo toads and bufotenine: fact and fiction surrounding an alleged psychedelic. J. Psychoactive Drugs. 1996;28(3):267–290. [PubMed]
14. Hill S.L, Thomas S.H. Clinical Toxicology of newer recreational drugs. Clin. Toxicol. 2011;49(8):705–719. doi: 10.3109/15563650.2011.615318. [PubMed] [Cross Ref]
15. Fantegrossi W.E, Murnane K.S, Reissig C.J. The behavioral pharmacology of hallucinogens. Biochem. Pharmacol. 2008;75(1):17–33. doi: 10.1016/j.bcp.2007.07.018. [PMC free article] [PubMed] [Cross Ref]
16. Nagai F, Nonaka R, Satoh Hisashi Kamimura K, Kaminura H. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur. J. Pharmacol. 2007;559(2-3):132–137. doi: 10.1016/j.ejphar.2006.11.075. [PubMed] [Cross Ref]
17. Ray T.S. Psychedelics and the human receptorome. 2010. [PMC free article] [PubMed] [Cross Ref]
18. Lessin AW, Long RF, Parkes MW. Central stimulant actions of á-alkyl substituted tryptamine in mice. Br. J. Pharmacol.Chemoter. 1965;24:49–67. [PMC free article] [PubMed]
19. Shulgin A.T, Shulgin A. TIHKAL: the continuation Tryptamines I have known and loved. Berkley: Transform Press; 1997.
20. Kamour A, James D, Spears R, Cooper G, Lupton D.J, Eddleston M, Thompson J.P, Vale A.J, Thanacoody H.K, Hill S.L, Thomas S.H. Patterns of presentation and clinical toxicity after reported use of alpha methyltryptamine in the United Kingdom. A report from the UK National Poisons Information Service. Clin. Toxicol. (Phila) 2014;52(3):192–197. doi: 10.3109/15563650.2014.885983. [PubMed] [Cross Ref]
21. Available from http://www.deadiversion.usdoj.gov/drug_chem_ info/amt.pdf . 2014. [Last access on 1th August].
22. Available from https://legal-high-inhaltsstoffe.de/sites/default/files/uploads/amt.pdf . 2014. [Last access on 1th August].
23. Wilcox J. Psychoactive properties of alpha-methyltryptamine: analysis from self reports of users. J. Psychoactive Drugs. 2012;44(3):274–276. doi: 10.1080/02791072.2012.704592. [PubMed] [Cross Ref]
24. Available from https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/318693/ 2014. [Last access on 5th August].
25. Available from http://www.sgul.ac.uk/research/projects/icdp/ourwork-programmes/pdfs/drd_ar_2013.pdf . 2014. [Last access on 11th August].
26. Huang X.M, Johnson M.P, Nichols D.E. Reduction in brain serotonin markers by alpha-ethyltryptamine (Monase) Eur. J. Pharmacol. 1991;200(1):187–190. doi: 10.1016/0014-2999(91)90686-K. [PubMed] [Cross Ref]
27. Greig M.E, Walk R.A, Gibbons A.J. The effect of three tryptamine derivatives on serotonin metabolism in vitro and in vivo. J. Pharmacol. Exp. Ther. 1959;127(2):110–115. [PubMed]
28. Ott J. Ayahuasca Analogues: Pangæan Entheogens. Kennewick, WA, USA: Natural Products; 1994. pp. 81–83.
29. Strassman R.J. Human psychopharmacology of N,N-dimethyltryptamine. Behav. Brain Res. 1996;73(1-2):121–124. doi: 10.1016/0166-4328(96)00081-2. [PubMed] [Cross Ref]
30. Gable RS. Risk assessment of ritual use of oral dimethyltryptamine (DMT) and harmala alkaloids. Addiction. 2007;102(1):24–34. [PubMed]
31. Szara S, Rockland L.H, Rosenthal D, Handlon J.H. Psychological effects and metabolism of N,N-diethyltryptamine in man. Arch. Gen. Psychiatry. 1966;15(3):320–329. doi: 10.1001/archpsyc.1966.01730150096014. [PubMed] [Cross Ref]
32. Winter J.C. Behavioral effects of N,N-diethyltryptamine: absence of antagonism by xylamidine tosylate. J. Pharmacol. Exp. Ther. 1969;169(1):7–16. [PubMed]
33. Available from: https://www.erowid.org/library/books_online/tihkal/tihkal03.shtml . [Last access on 21th August, 2014].
34. Thiagaraj H.V, Russo E.B, Burnett A, Goldstein E, Thompson C.M, Parker K.K. Binding properties of dipropyltryptamine at the human 5-HT1a receptor. Pharmacology. 2005;74(4):193–199. doi: 10.1159/000085649. [PubMed] [Cross Ref]
35. Grof S, Soskin R.A, Richards W.A, Kurland A.A. DPT as an adjunct in psychotherapy of alcoholics. Int. Pharmacopsychiatry. 1973;8(1):104–115. [PubMed]
36. Carbonaro T.M, Forster M.J, Gatch M.B. Discriminative stimulus effects of N,N-diisopropyltryptamine. Psychopharmacology (Berl.) 2013;226(2):241–246. doi: 10.1007/s00213-012-2891-x. [PMC free article] [PubMed] [Cross Ref]
37. Gatch M.B, Forster M.J, Janowsky A, Eshleman A.J. Abuse Liability Profile of Three Substituted Tryptamines. 2011. [PMC free article] [PubMed] [Cross Ref]
38. Nichols D.E. Hallucinogens. Pharmacol. Ther. 2004;101(2):131–181. doi: 10.1016/j.pharmthera.2003.11.002. [PubMed] [Cross Ref]
39. Shulgin A.T, Carter M.F. N, N-Diisopropyltryptamine (DIPT) and 5-methoxy-N,N-diisopropyltryptamine (5-MeO-DIPT). Two orally active tryptamine analogs with CNS activity. Commun. Psychopharmacol. 1980;4(5):363–369. [PubMed]
40. Bertol E, Mari F, Lodi F, Marozzi E. Trattato di Tossicologia Forense. CEDAM; 2000. p. 471.
41. Lindenblatt H, Krämer E, Holzmann-Erens P, Gouzoulis-Mayfrank E, Kovar K.A. Quantitation of psilocin in human plasma by high-performance liquid chromatography and electrochemical detection: comparison of liquid-liquid extraction with automated on-line solid-phase extraction. J. Chromatogr. B Biomed. Sci. Appl. 1998;709(2):255–263. doi: 10.1016/S0378-4347(98)00067-X. [PubMed] [Cross Ref]
42. Halpern J.H. Hallucinogens and dissociative agents naturally growing in the United States. Pharmacol. Ther. 2004;102(2):131–138. doi: 10.1016/j.pharmthera.2004.03.003. [PubMed] [Cross Ref]
43. Grieshaber A.F, Moore K.A, Levine B. The detection of psilocin in human urine. J. Forensic Sci. 2001;46(3):627–630. [PubMed]
44. Peden NR, Macaulay KEC, Bisset AF, Crooks J, Pelosi AJ. Clinical toxicology of 'magic mushroom' ingestion. Postgrad. Med. J. 1981;57(671):543–545. [PMC free article] [PubMed]
45. Hasler F, Grimberg U, Benz M.A, Huber T, Vollenweider F.X. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology (Berl.) 2004;172(2):145–156. doi: 10.1007/s00213-003-1640-6. [PubMed] [Cross Ref]
46. Available from http://www.erowid.org/experiences/exp.php? ID=50758 . 2014. [Last access on 25th August].
47. Ujváry, I. Psychoactive natural products: overview of recent developments. [Last access on 25th August];Ann. Ist. Super. Sanita. 2014 50(1):12–27. doi: 10.4415/ANN_14_01_04. [PubMed] [Cross Ref]
48. Moffat A.C, Osselton M.D, Widdop B, Watts J. Clarke’s Analysis of Drugs and Poisons. 4th ed. Pharmaceutical Press; 2011. pp. 1004–1005.
49. Fuller R.W, Snoddy H.D, Perry K.W. Tissue distribution, metabolism and effects of bufotenine administered to rats. Neuropharmacology. 1995;34(7):799–804. doi: 10.1016/0028-3908(95)00049-C. [PubMed] [Cross Ref]
50. Ott J. Pharmañopo-psychonautics: human intranasal, sublingual, intrarectal, pulmonary and oral pharmacology of bufotenine. J. Psychoactive Drugs. 2001;33(3):273–281. doi: 10.1080/02791072.2001.10400574. [PubMed] [Cross Ref]
51. Available from http://www.erowid.org/chemicals/5meo_amt/5meo_amt_info2.shtml . 2014. [Last access on 25th August].
52. Glennon RA, Chaurasia C, Titeler M. Binding of indolylalkylamines at 5-HT2 serotonin receptors examination of a hydrophobic binding region. J. Med. Chem. 1990;33(10):2777– 2784. [PubMed]
53. Tomaszewski Z, Johnson M.P, Huang X, Nichols D.E. Benzofuran bioisosteres of hallucinogenic tryptamines. J. Med. Chem. 1992;35(11):2061–2064. doi: 10.1021/jm00089a017. [PubMed] [Cross Ref]
54. Available from http://www.erowid.org/chemicals/5meo_amt/ 5meo_amt_info1.shtml . 2014. [Last access on 25th August].
55. Available from http://www.erowid.org/chemicals/lsd/lsd_media2.shtml . 2014. [Last access on 25th August].
56. Pachter IJ, Zacharias DE, Ribeiro O. Indole alkaloids of acer saccharinum (the Silver Maple) Dictyoloma incanescens Piptadenia colubrina. and Mimosa hostilis. 1959:1285–1287.
57. Shen H.W, Jiang X.L, Winter J.C, Yu A.M. Psychedelic 5-methoxy-N,N-dimethyltryptamine: metabolism, pharmacokinetics, drug interactions, and pharmacological actions. Curr. Drug Metab. 2010;11(8):659–666. doi: 10.2174/138920010794233495. [PMC free article] [PubMed] [Cross Ref]
58. Ott J. Pharmepéna-Psychonautics: Human intranasal, sublingual and oral pharmacology of 5-methoxy-N,N-dimethyl-tryptamine. J. Psychoactive Drugs. 2001;33(4):403–407. doi: 10.1080/02791072.2001.10399925. [PubMed] [Cross Ref]
59. Sogawa C, Sogawa N, Tagawa J, Fujino A, Ohyama K, Asanuma M, Funada M, Kitayama S. 5-Methoxy-N,N-diisopropyltryptamine (Foxy), a selective and high affinity inhibitor of serotonin transporter. Toxicol. Lett. 2007;170(1):75–82. doi: 10.1016/j.toxlet.2007.02.007. [PubMed] [Cross Ref]
60. Muller AA. New drugs of abuse update Foxy Methoxy. J. Emerg. Nurs. 2004;30(5):507–508. [PubMed]
61. Fantegrossi W.E, Harrington A.W, Kiessel C.L, Eckler J.R, Rabin R.A, Winter J.C, Coop A, Rice K.C, Woods J.H. Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol. Biochem. Behav. 2006;83(1):122–129. doi: 10.1016/j.pbb.2005.12.015. [PubMed] [Cross Ref]
62. Tanaka E, Kamata T, Katagi M, Tsuchihashi H, Honda K. A fatal poisoning with 5-methoxy-N,N-diisopropyltryptamine, Foxy. Forensic Sci. Int. 2006;163(1-2):152–154. doi: 10.1016/j.forsciint.2005.11.026. [PubMed] [Cross Ref]
63. Nonaka R, Nagai F, Ogata A, Satoh K. In vitro screening of psychoactive drugs by [35S] GTPgammaS binding in rat brain membranes. Biol. Pharm. Bull. 2007;30(12):2328–2333. doi: 10.1248/bpb.30.2328. [PubMed] [Cross Ref]
64. Available from http://www.erowid.org/chemicals/5meo_mipt/ 5meo_mipt_effects.shtml . 2014. [Last access on 25th August].
65. Al-Assmar S.E. The seeds of the Hawaiian baby woodrose are a powerful hallucinogen. Arch. Intern. Med. 1999;159(17):2090. doi: 10.1001/archinte.159.17.2090. [PubMed] [Cross Ref]
66. Available from https://www.erowid.org/plants/hbw/hbw_effects. shtml . 2014. [Last access on 25th August].
67. Available from https://www.erowid.org/plants/morning_glory/morning_glory.shtml . 2014. [Last access on 25th August].
68. Available from http://www.ehow.com/about_5073540_psychedeliceffects-morning-glory-seeds.html . 2014. [Last access on 25th August].
69. Available from http://www.erowid.org/library/books_online/tihkal/tihkal48.shtml . 2014. [Last access on 25th August].
70. Available from https://www.erowid.org/plants/hbw/hbw_effects. shtml . 2014. [Last access on 25th August].
71. Available from https://www.erowid.org/plants/hbw/hbw_effects. shtml . 2014. [Last access on 25th August].
72. Fantegrossi W.E, Reissig C.J, Katz E.B, Yarosh H.L, Rice K.C, Winter J.C. Hallucinogen-like effects of N,N-dipropyltryptamine (DPT): possible mediation by serotonin 5-HT1A and 5-HT2A receptors in rodents. Pharmacol. Biochem. Behav. 2008;88(3):358–365. doi: 10.1016/j.pbb.2007.09.007. [PMC free article] [PubMed] [Cross Ref]
73. Sitaram BR, Lockett L, Talomsin R, Blackman GL, McLeod WR. In vivo metabolism of 5-methoxy-N N-dimethyltryptamine and N N-dimethyltryptamine in the rat. Biochem. Pharmacol. . 1987;36(9):1509–1512. [PubMed]
74. Available from: http://www.erowid.org/experiences/exp_front.shtml . [Last access on 26th August, 2014].
75. Available from: http://www.erowid.org/library/books_online/tihkal/tihkal.shtml#index . [Last access on 26th August, 2014].
76. Dargan P.I, Wood D.M. Novel Psychoactive Substances: Classification, Pharmacology and Toxicology. Elsevier; 2013.
77. Available from: http://www.erowid.org/chemicals/5meo_dalt/5meo_dalt_info1.shtml . [Last access on 26th August, 2014].
78. Available from: http://www.erowid.org/chemicals/ [Last access on 26th August, 2014].
79. Available from: http://www.erowid.org/chemicals/lsa/lsa_effects.shtml . [Last access on 26th August, 2014].
80. Wood D.M, Conran P, Dargan P.I. ICD-10 coding: poor identification of recreational drug presentations to a large emergency department. Emerg. Med. J. 2011;28(5):387–389. doi: 10.1136/emj.2009.088344. [PubMed] [Cross Ref]
81. Holstege C.P, Baer A.B, Kirk M.A. Prolonged hallucinations following ingestion of alpha-methyl-tryptamine. J. Toxicol. Clin. Toxicol. 2003;41(5):641–752. doi: 10.1081/clt-120030949. [Cross Ref]
82. Long H, Hoffman R.S, Nelson L.S. Alpha-methyltryptamine revisited due to easy internet access; EAPCCT XXIII International Congress; 2003.
83. Dailey R.M, Nelson L.D, Scaglione J.M. Tachycardia and rhabdomyolysis after intentional ingestion of N,N-Dipropyltryptamine. J. Toxicol. Clin. Toxicol. 2003;41(5):742–743.
84. Brush D.E, Bird S.B, Boyer E.W. Monoamine oxidase inhibitor poisoning resulting from Internet misinformation on illicit substances. J. Toxicol. Clin. Toxicol. 2004;42(2):191–195. doi: 10.1081/CLT-120030949. [PubMed] [Cross Ref]
85. Wilson J.M, McGeorge F, Smolinske S, Meatherall R. A foxy intoxication. Forensic Sci. Int. 2005;148(1):31–36. doi: 10.1016/j.forsciint.2004.04.017. [PubMed] [Cross Ref]
86. Alatrash G, Majhail N.S, Pile J.C. Rhabdomyolysis after ingestion of “Foxy”, a hallucinogenic tryptamine derivative. Mayo Clin. Proc. 2006;81(4):550–551. [PubMed]
87. Smolinske S.C, Rastogi R, Schenkel S. Foxy methoxy: a new drug of abuse. J. Med. Toxicol. 2005;1(1):22–25. doi: 10.1007/BF03160901. [PubMed] [Cross Ref]
88. Meatherall R, Sharma P. Foxy, a designer tryptamine hallucinogen. J. Anal. Toxicol. 2003;27(5):313–317. doi: 10.1093/jat/27.5.313. [PubMed] [Cross Ref]
89. Available from: http://www.aapcc.org/annual-reports/ [Last access on 26th August, 2014].
90. Shimizu E, Watanabe H, Kojima T, Hagiwara H, Fujisaki M, Miyatake R, Hashimoto K, Iyo M. Combined intoxication with methylone and 5-MeO-MIPT. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31(1):288–291. doi: 10.1016/j.pnpbp.2006.06.012. [PubMed] [Cross Ref]
91. Jovel A, Felthous A, Bhattacharyya A. Delirium due to intoxication from the novel synthetic tryptamine 5-MeO-DALT. J. Forensic Sci. 2014;59(3):844–846. doi: 10.1111/1556-4029.12367. [PubMed] [Cross Ref]
92. Boland D.M, Andollo W, Hime G.W, Hearn W.L. Fatality due to acute α-methyltryptamine intoxication. J. Anal. Toxicol. 2005;29(5):394–397. doi: 10.1093/jat/29.5.394. [PubMed] [Cross Ref]
93. Butin J.W. Agranulocytosis following Monase therapy. J. Kans. Med. Soc. 1962;63:338–340. doi: 10.1016/0002-9343(86)90274-3. [PubMed] [Cross Ref]
94. Morano R.A, Spies C, Walker F.B, Plank S.M. Fatal intoxication involving etryptamine. J. Forensic Sci. 1993;38(3):721–725. [PubMed]
95. Daldrup T, Heller C, Matthiesen U, Honus S, Bresges A, Haarhoff K. Etryptamine, a new designer drug with a fatal effect. Z. Rechtsmed. 1986;97(1):61–68. doi: 10.1007/BF00200960. [PubMed] [Cross Ref]
96. Sklerov J, Levine B, Moore K.A, King T, Fowler D. A fatal intoxication following the ingestion of 5-methoxy-N,N-dimethyltryptamine in an ayahuasca preparation. J. Anal. Toxicol. 2005;29(8):838–841. doi: 10.1093/jat/29.8.838. [PubMed] [Cross Ref]
97. Available from: http://www.erowid.org/chemicals/5meo_dipt/5meo_dipt_media1.shtml . [Last access on 26th August, 2014].
98. Hylin J.W, Watson D.P. Ergoline Alkaloids in Tropical Wood Roses. Science. 1965;148(3669):499–500. doi: 10.1126/science.148.3669.499. [PubMed] [Cross Ref]
99. Available from: https://www.erowid.org/experiences/exp.php?ID=7110 . [Last access on 26th August, 2014].
100. Göpel C, Maras A, Schmidt M.H. [Hawaiian baby rose wood: case report of an argyreia nervosa induced toxic psychosis] Psychiatr. Prax. 2003;30(4):223–224. doi: 10.1055/s-2003-39490. [PubMed] [Cross Ref]
101. Montesano C, Sergi M, Moro M, Napoletano S, Romolo F.S, Del Carlo M, Compagnone D, Curini R. Screening of methylenedioxyamphetamine- and piperazine-derived designer drugs in urine by LC-MS/MS using neutral loss and precursor ion scan. J. Mass Spectrom. 2013;48(1):49–59. doi: 10.1002/jms.3115. [PubMed] [Cross Ref]
102. Strano-Rossi S, Anzillotti L, Castrignanò E, Romolo F.S, Chiarotti M. Ultra high performance liquid chromatography-electrospray ionization-tandem mass spectrometry screening method for direct analysis of designer drugs, “spice” and stimulants in oral fluid. J. Chromatogr. A. 2012;1258:37–42. doi: 10.1016/j.chroma.2012.07.098. [PubMed] [Cross Ref]
103. Favretto D, Pascali J.P, Tagliaro F. New challenges and innovation in forensic toxicology: focus on the “New Psychoactive Substances” J. Chromatogr. A. 2013;1287:84–95. doi: 10.1016/j.chroma.2012.12.049. [PubMed] [Cross Ref]
104. Vorce S.P, Sklerov J.H. A general screening and confirmation approach to the analysis of designer tryptamines and phenethylamines in blood and urine using GC-EI-MS and HPLC-electrospray-MS. J. Anal. Toxicol. 2004;28(6):407–410. doi: 10.1093/jat/28.6.407. [PubMed] [Cross Ref]
105. Wohlfarth A, Weinmann W, Dresen S. LC-MS/MS screening method for designer amphetamines, tryptamines, and piperazines in serum. Anal. Bioanal. Chem. 2010;396(7):2403–2414. doi: 10.1007/s00216-009-3394-4. [PubMed] [Cross Ref]
106. Meyer M.R, Caspar A, Brandt S.D, Maurer H.H. A qualitative/quantitative approach for the detection of 37 tryptamine-derived designer drugs, 5 β-carbolines, ibogaine, and yohimbine in human urine and plasma using standard urine screening and multi-analyte approaches. Anal. Bioanal. Chem. 2014;406(1):225–237. doi: 10.1007/s00216-013-7425-9. [PubMed] [Cross Ref]
107. Chen B.H, Liu J.T, Chen W.X, Chen H.M, Lin C.H. A general approach to the screening and confirmation of tryptamines and phenethylamines by mass spectral fragmentation. Talanta. 2008;74(4):512–517. doi: 10.1016/j.talanta.2007.06.012. [PubMed] [Cross Ref]
108. Ishida T, Kudo K, Kiyoshima A, Inoue H, Tsuji A, Ikeda N. Sensitive determination of alpha-methyltryptamine (AMT) and 5-methoxy-N,N-diisopropyltryptamine (5MeO-DIPT) in whole blood and urine using gas chromatography-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005;823(1):47–52. doi: 10.1016/j.jchromb.2004.10.067. [PubMed] [Cross Ref]
109. Pichini S, Pujadas M, Marchei E, Pellegrini M, Fiz J, Pacifici R, Zuccaro P, Farré M, de la Torre R. Liquid chromatography-atmospheric pressure ionization electrospray mass spectrometry determination of “hallucinogenic designer drugs” in urine of consumers. J. Pharm. Biomed. Anal. 2008;47(2):335–342. doi: 10.1016/j.jpba.2007.12.039. [PubMed] [Cross Ref]
110. Helander A, Bäckberg M, Hultén P, Al-Saffar Y, Beck O. Detection of new psychoactive substance use among emergency room patients: results from the Swedish STRIDA project. Forensic Sci. Int. 2014;243:23–29. doi: 10.1016/j.forsciint.2014.02.022. [PubMed] [Cross Ref]
111. Al-Saffar Y, Stephanson N.N, Beck O. Multicomponent LC-MS/MS screening method for detection of new psychoactive drugs, legal highs, in urine-experience from the Swedish population. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013;930:112–120. doi: 10.1016/j.jchromb.2013.04.043. [PubMed] [Cross Ref]
112. Katagi M, Kamata T, Zaitsu K, Shima N, Kamata H, Nakanishi K, Nishioka H, Miki A, Tsuchihashi H. Metabolism and toxicologic analysis of tryptamine-derived drugs of abuse. Ther. Drug Monit. 2010;32(3):328–331. doi: 10.1097/FTD.0b013e3181dcb40c. [PubMed] [Cross Ref]
113. Wang M.J, Liu J.T, Chen H.M, Lin J.J, Lin C.H. Comparison of the separation of nine tryptamine standards based on gas chromatography, high performance liquid chromatography and capillary electrophoresis methods. J. Chromatogr. A. 2008;1181(1-2):131–136. doi: 10.1016/j.chroma.2007.12.031. [PubMed] [Cross Ref]
114. Strano Rossi S, Odoardi S, Gregori A, Peluso G, Ripani L, Ortar G, Serpelloni G, Romolo F.S. An analytical approach to the forensic identification of different classes of new psychoactive substances (NPSs) in seized materials. Rapid Commun. Mass Spectrom. 2014;28(17):1904–1916. doi: 10.1002/rcm.6969. [PubMed] [Cross Ref]
115. Hugel J, Meyers F.A, Lankin D.C. Analysis of the hallucinogens. In: Smith F.P, editor. Handbook of forensic drug analysis. 2005. pp. 153–234.

